Mini AMES Test
Genetic safety assessment is extremely important in the development of pharmaceuticals. Compounds that induce genetic mutations at preclinical models are often discontinued for further efforts due to the dramatically increased risk of toxicity. The only rare exception is certain life-saving oncology drug candidates.
Quintara Discovery offers non-GLP mini Ames tests in which Salmonella cell lines are used to monitor the mutagenic potential of the test compounds. Basically these Salmonella strains cannot grow in histidine-free medium unless mutations occur in certain genes.
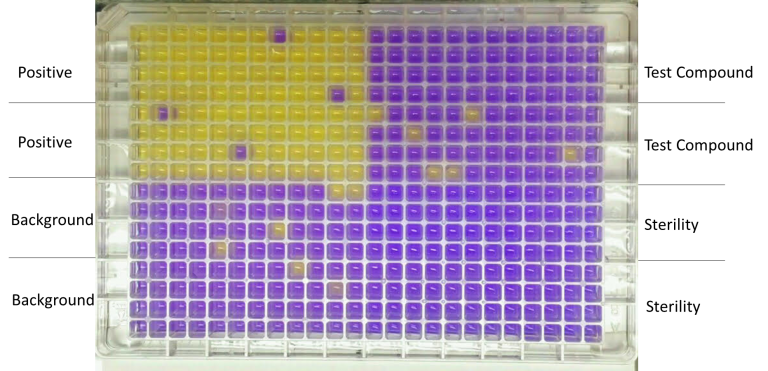
The mini Ames assay is performed in 384-well plates using two Salmonella strains: TA98 (frameshift mutation) and TA100 (base-pair substitutions). After 48-72 hour incubation with test articles, the bacterial growth is measured spectrophotometrically using a pH indicator that changes color in response to the bacterial growth (example shown below). Positive, background and sterile controls are always included. The assay is performed in at least 48 wells for each condition.
The Ames test can be carried out in the presence and absence of a metabolizing system (e.g., Aroclor 1254-induced rat liver S9 fraction) to identify potential mutagenicity by the parent compound and its metabolites.